Demystifying the Regulatory Environment (An IO POV)
Introduction: The Food and Drug Administration (FDA) plays a crucial role in ensuring the safety and efficacy of drugs, medical devices, and other healthcare products available to the public. This regulatory process is a rigorous and complex journey that aims to protect patient health while promoting innovation. In this blog post, we will delve into the FDA regulatory process, highlighting its key components and the steps involved.
Preclinical Testing: Before any new drug or medical device can be tested on humans, extensive preclinical testing is conducted. This phase involves laboratory and animal studies to evaluate the product's safety profile, potential benefits, and potential risks. These tests generate crucial data that inform subsequent clinical trials.
Investigational New Drug (IND) Application: Once preclinical testing demonstrates promising results, the next step is to submit an IND application to the FDA. This application includes comprehensive data from preclinical studies, proposed clinical trial protocols, and information about the manufacturing processes. The FDA reviews the IND application to ensure patient safety, proper trial design, and adherence to ethical guidelines.
Clinical Trials: Clinical trials are conducted in several phases to assess the safety, efficacy, and optimal dosage of the investigational product. Phase 1 trials involve a small number of healthy volunteers to determine safety and dosage range. Phase 2 trials expand the study population to evaluate effectiveness and potential side effects. Phase 3 trials involve larger groups and compare the investigational product with existing treatments. These trials provide critical evidence for the FDA's evaluation.
New Drug Application (NDA) or Biologics License Application (BLA): After successful completion of clinical trials, the sponsor submits an NDA or BLA to the FDA. This comprehensive application includes data from preclinical and clinical studies, manufacturing details, labeling information, and proposed usage guidelines. The FDA conducts an extensive review, considering factors such as efficacy, safety, manufacturing quality, and labeling accuracy.
FDA Review and Decision: During the review process, the FDA assesses all submitted data to ensure that the benefits of the product outweigh its potential risks. The agency carefully evaluates clinical trial results, manufacturing processes, labeling, and proposed indications for use. This thorough evaluation aims to protect public health by approving only safe and effective products.
Post-Market Monitoring: Even after approval, the FDA continues to monitor the product's safety and effectiveness through post-market surveillance. Adverse events and side effects are tracked, and the FDA collaborates with healthcare professionals and manufacturers to address any emerging concerns. Regular inspections of manufacturing facilities are conducted to maintain quality standards.
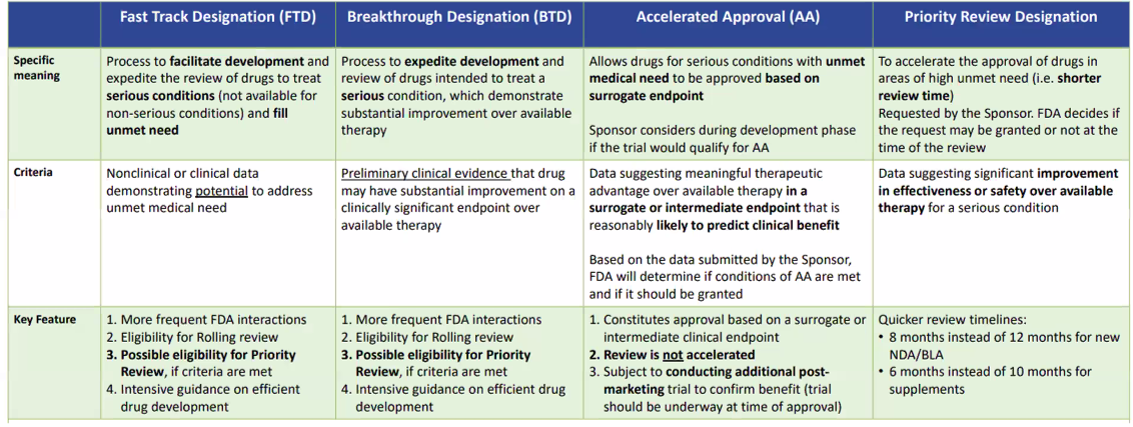
Fast Track Designation vs. Breakthrough Therapy vs Accelerated Approval vs Priority Reivew:
a) Fast Track Designation: Fast Track designation is a program established by the FDA to expedite the development and review of drugs that address unmet medical needs for serious conditions. This designation is intended to accelerate the availability of promising treatments to patients. To qualify for Fast Track designation, a drug must demonstrate the potential to provide significant improvement over existing therapies or address an urgent medical need.
Benefits of Fast Track designation include more frequent communication and collaboration between the FDA and the drug developer, including the opportunity for rolling reviews, which allow for the submission of sections of the marketing application as they are completed rather than waiting for the entire application to be finished. Additionally, Fast Track designation may allow for priority review, which can shorten the review time by several months compared to standard review timelines.
b) Breakthrough Therapy Designation: Breakthrough Therapy designation is another program initiated by the FDA, specifically for drugs that demonstrate substantial improvement over existing treatments for serious or life-threatening conditions. The designation aims to expedite the development and review process by providing enhanced support and guidance to drug developers.
To qualify for Breakthrough Therapy designation, preliminary clinical evidence must indicate that the drug has the potential to offer significant improvement in at least one clinically meaningful endpoint compared to available therapies. This designation is typically granted based on early-stage trial data.
Breakthrough Therapy designation offers benefits similar to Fast Track designation, including increased interaction and guidance from the FDA throughout the development and review process. Additionally, Breakthrough Therapy-designated drugs may be eligible for accelerated approval, which allows for the approval of drugs based on surrogate endpoints that are reasonably likely to predict clinical benefit, reducing the time required for traditional endpoints such as overall survival.
a) Accelerated Approval (AA): Accelerated Approval is a pathway provided by the FDA to expedite the availability of drugs for serious or life-threatening conditions that fill an unmet medical need. This pathway allows for the approval of drugs based on surrogate endpoints or intermediate clinical trial outcomes that are reasonably likely to predict clinical benefit. These surrogate endpoints are measures that are considered likely to predict clinical benefit, such as tumor size reduction or improvement in a laboratory marker, rather than the ultimate clinical endpoint like overall survival.
Under the Accelerated Approval program, drugs can receive initial approval based on these surrogate endpoints, with the requirement that the drug developer conducts further studies to confirm the anticipated clinical benefit. This post-approval confirmatory trial is typically conducted in a larger patient population, and its results will determine whether the drug maintains its approval.
Accelerated Approval provides an avenue for patients to access promising therapies earlier, while ensuring that ongoing research verifies the drug's clinical benefits. This program promotes the development of innovative treatments for severe conditions with limited treatment options.
b) Priority Review Designation: Priority Review is a designation given to certain drugs that provide significant improvements in safety or effectiveness compared to existing treatments. It expedites the review process, reducing the standard review time from ten months to six months.
To qualify for Priority Review, a drug must address an unmet medical need or offer a substantial improvement in efficacy or safety for a condition with available treatments. The FDA assigns a multidisciplinary review team to evaluate the drug application and provides increased guidance and communication to streamline the review process.
Priority Review designation recognizes the urgency of bringing innovative therapies to patients and ensures that potentially life-saving or life-enhancing drugs receive prompt evaluation, accelerating their availability in the market.
Conclusion: The FDA regulatory process is a critical framework that safeguards public health by ensuring that drugs, medical devices, and other healthcare products meet stringent safety and efficacy standards. The process involves rigorous preclinical testing, well-designed clinical trials, comprehensive applications, and thorough FDA review. This multifaceted approach helps bring innovative treatments to patients while prioritizing their well-being. By maintaining post-market surveillance, the FDA ensures that approved products remain safe and effective throughout their lifecycle. Ultimately, this regulatory process provides confidence and peace of mind to healthcare professionals and patients alike.